Masculinizing Hormone Therapy
The cornerstone of hormone therapy for trans masculine patients is testosterone. The goal of treatment is virilization – the development of masculine secondary sexual characteristics.
Quick reference guide for masculinizing hormone therapy:
Quick reference guide for primary care providers (ENGLISH VERSION)
Aide-mémoire pour professionnels de la santé de première ligne (FRENCH VERSION)
-
Topics in this section:
- Hormonal agents
- Expected effects
- Risk mitigation
- Monitoring strategies
- Long-term preventive care
Hormonal agents
Testosterone
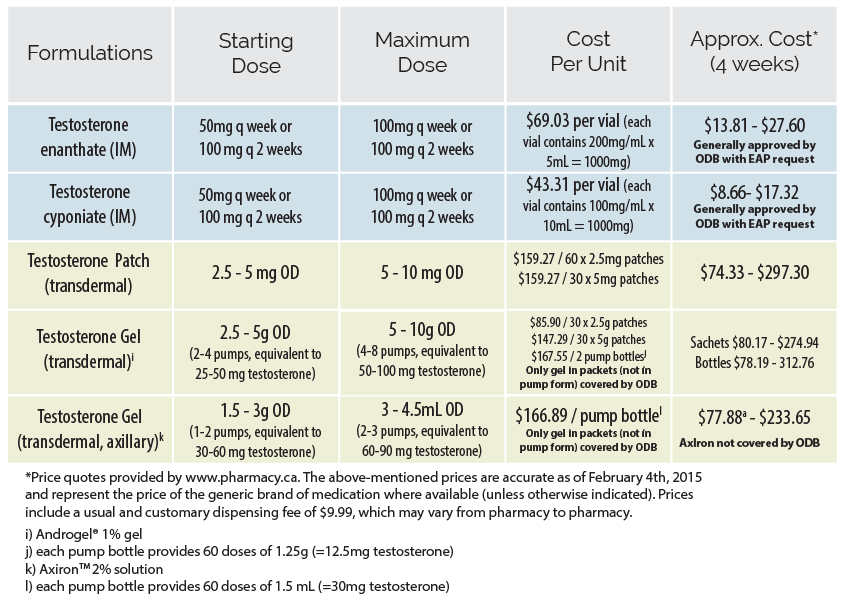
In Ontario, options for testosterone administration include injectable and transdermal preparations (patch or gel). Injectable formulations are most commonly used, due to their superior efficacy and affordability. While intramuscular injection (IM) is the most common means of administering parenteral testosterone, subcutaneous (SC) delivery has also been used with clinical efficacy and is very well-tolerated. Proponents describe less discomfort for patients, a decreased rate of injection-site complications, and increased capacity for self-injection. An initial dose reduction of 10-15% can be considered if switching from IM to SC.
Keep in mind
If patients wish to self-inject, it is important to instruct them on the technique for safe injection and sharps disposal. A written step-by-step guide on self-injection for patients is available from Fenway Health| Formulations | Starting/Low Dose | Maximum Dose | Cost per unit | Approx Cost*(4 weeks) |
|---|---|---|---|---|
| Testosterone enanthatea (IM/SC) | 20 - 50 mg q weekly or 40 - 100 mg q 2 weeks | 100 mg q weekly or 200 mg q 2 weeks | $73.50 per 5 mL vial (each vial contains 200 mg/mL x 5mL = 1000 mg) | $14 - $29 Generally covered by ODB with EAP request |
| Testosterone Cypionate (IM/SC)a | $64 per 10 mL vial (each vial contains 100mg/mL x 10mL =1000 mg) | $13 - $26 Generally covered by ODB with EAP request |
||
| Testosterone (transdermal) Patchb | 2.5 – 5 mg daily | 5 - 10 mg daily | $164/60 x 2.5 mg patches $169/30 x 5 mg patches | $76.50 - $315 |
| Testosterone Gel 1% (transdermal) | 2.5 - 5 g daily (2-4 pumps, equivalent to 25 - 50 mg testosterone) | 5 - 10 g daily (4-8 pumps, equivalent to 50 - 100 mg testosterone) | $67/30 x 2.5 g sachets $110 / 30 x 5 g sachets $175 / 2 pump bottlesc | Sachets:
$62 - $205 Bottles: $81 - $32 |
References and Notes
|
||||
Expected effects
What to expect from testosterone
The degree and rate of physical effects is dependent on the dose and route of administration, as well as patient-specific factors such as age, genetics, body habitus and lifestyle.
Desired androgenic effects of testosterone therapy include deepened voice, cessation of menses, clitoral growth, increased muscle mass, and hair growth in androgen dependent areas including facial hair. Breast tissue may lose glandularity, but generally does not lose mass or hemi circumference.1 Typically, patients taking testosterone will experience masculinizing changes over a period of months to years. The timeframe of physiologic changes may be slightly slower with the use of transdermal preparations.
Sources
- 1Futterweit W, Schwartz IS. Histopathology of the breasts of 12 women receiving long-term exogenous androgen therapy. The Mount Sinai journal of medicine 1988;55(4):309-312.
Keep in mind
Testosterone therapy results in both reversible and irreversible masculinization.Effects and expected time course
Hover over the coloured regions to view expected information on the reversibility, onseta and maximum effectsa of physical changes
Skin changes
Skin oiliness/acne
- Reversibility:
- Reversible
- Expected onset:
- 1 - 6 months
- Expected max. effect:
- 1 - 2 years
Body and facial hair growth
Facial hair grows and body hair thickens.
- Reversibility:
- Irreversible
- Expected onset:
- 3 - 6 months
- Expected max. effect:
- 4 - 5 years
Scalp hair loss
Highly dependant on age and inheritance; May be minimal
- Reversibility:
- Irreversible
- Expected onset:
- 6 - 12 months b
- Expected max. effect:
- Variable
Deepened voice
- Reversibility:
- Irreversible
- Expected onset:
- 6 - 12 months
- Expected max. effect:
- 1 - 2 years
Cessation of menses
- Reversibility:
- Reversible
- Expected onset:
- 1 - 6 months
- Expected max. effect:
- n/a
Clitoral enlargement
- Reversibility:
- Irreversible
- Expected onset:
- 3 - 6 months
- Expected max. effect:
- 1 - 2 years
Vaginal atrophy
- Reversibility:
- Reversible
- Expected onset:
- 1 - 6 months
- Expected max. effect:
- 1 - 2 years
Infertility
- Reversibility:
- Variable
- Expected onset:
- Variable
- Expected max. effect:
- Variable
Increased muscle mass/strengthc
Significantly dependent on amount of exercise
- Reversibility:
- Reversible
- Expected onset:
- 6 - 12 months
- Expected max. effect:
- 2 - 5 years
Body fat redistribution
Fat redistributes from buttock/hip/thigh regions to the abdomen and mid-section.
- Reversibility:
- Reversible/
Variable - Expected onset:
- 1 - 6 months
- Expected max. effect:
- 2 - 5 years
Sources
- a) Estimates for onset and expected maximum effect represent published and unpublished clinical observations. Sources:
- Gooren LJ, Harmsen-Louman W, van Kessel H. Followup of prolactin levels in long-term oestrogen-treated male-to-female transsexuals with regard to prolactinoma induction. Clin Endocrinol (Oxf). 1985;22(2):201–207.
- Toorians AWFT, Thomassen MCLGD, Zweegman S, Magdeleyns EJP, Tans G, Gooren LJG, et al. Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people. J Clin Endocrinol Metab. 2003 Dec;88(12):5723–9.
- Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, Kaufman JM, T’Sjoen G. Crosssex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11(8):1999–2011.
- Asscheman H, Gooren LJ, Assies J, Smits JP, de Slegte R. Prolactin levels and pituitary enlargement in hormone-treated male-tofemale transsexuals.
- b) Significantly dependent on amount of exercise
- c) Highly dependent on age and inheritance; may be minimal
- Visual reference: Tetzlaff K. Patient’s guide to transgender, trans, & gender diverse health. 2015.
Risk mitigation
Absolute contraindications
- Pregnancy or breast feeding
- Active sex hormone-sensitive cancer (e.g., breast, endometrial)
- Unstable ischemic cardiovascular disease
- Poorly controlled psychosis or acute homicidality
- Psychiatric conditions which limit the ability to provide informed consent
- Hypersensitivity to one of the components of the formulation
Precautions and Risk mitigation
Pre-existing medical conditions and risk factors may impart increased risks with testosterone administration and should be considered in order to enable individualized discussions with patients regarding the risks and benefits of treatment. Available measures to reduce associated risks should be considered and discussed with patients and if possible, undertaken prior to or concurrently with the initiation of hormone therapy.
Select area of concern below
Neurologic
| Risk factors | How to minimize risks |
|---|---|
| Migraines | Consider daily migraine prophylaxis, consider transdermal route of administration |
| Androgen-sensitive epilepsy | Refer to neurology |
Endocrine
For more information on metabolic effects please refer to the full Guidelines.
| Risk factors | How to minimize risks |
|---|---|
| Uncontrolled diabetes | Identify and address barriers to optimal glycemic control, refer to dietitian, encourage lifestyle modification, initiate antiglycemic agent(s) per national guidelines, consider endocrinology referral, consider cardiac stress test, consider low dose/slow titration with monitoring |
| Uncontrolled dyslipidemia | Identify and address barriers to optimal lipid control, refer to dietitian, initiate anti-lipemic pharmacologic therapy per national guidelines, consider endocrinology referral, consider cardiac stress test, consider low dose/slow titration with monitoring |
Cardiovascular
For more information on cardiovascular disease please refer to the full Guidelines.
| Risk factors | How to minimize risks |
|---|---|
| Stable ischemic cardiovascular disease | Consider referral to cardiology, ensure optimal medical (including prophylactic anti-platelet agent(s) if indicated per national guidelines) and/or surgical management as indicated, risk factor optimization, consider transdermal route of administration, and/or low dose/slow titration with monitoring |
| Uncontrolled high blood pressure | Identify and address barriers to optimal BP control, initiate antihypertensive(s) as needed, consider cardiac stress test, consider low dose/slow titration with monitoring, consider referral to cardiology |
Hepatic
Elevation of liver enzymes may occur with testosterone therapy. For more information on hepatic dysfunction please refer to the full Guidelines.
| Risk factor | How to minimize risks |
|---|---|
| Hepatic Dysfunction | Dependent on etiology, e.g. minimize alcohol consumption, weight loss in NAFLD, consider referral to hepatology/GI, consider low dose/slow titration with monitoring |
| Hepatitis C | Screen patients who are at risk and treat as per current national guidelines
!
|
Gynaecologic
For more information on vaginal bleeding and endometrial cancer please refer to the full Guidelines.
| Risk factors | How to minimize risks |
|---|---|
| Inter-menstrual bleeding | Work up per national guidelines, gynaecology referral as needed |
| Oligo-/amenorrhea | Consider pelvic ultrasound (transvaginal if possible), consider progesterone-induced menstrual bleed prior to testosterone initiation |
Hematologic
| Risk factors | How to minimize risks |
|---|---|
| Polycythemia | Identify etiology and address contributing factors, consider referral to hematology, consider transdermal route of administration, and/or low dose/slow titration with monitoring. More information can be found in the full Guidelines |
| History of deep vein thrombosis (DVT), pulmonary embolism (PE) or hypercoagulable state | Identify and minimize existent risk factors, prophylactic anti-coagulation if indicated per current national guidelines, consider referral to hematology/thrombosis clinic, consider transdermal route of administration, and/or low dose/slow titration with monitoring |
Respiratory
| Risks/Precautions | How to minimize risks |
|---|---|
| Smoker | Encourage and support smoking cessation, consider referral to smoking cessation program/offer NRT and/or bupropion/varenicline, or negotiate a decrease in smoking, consider cardiac stress test. In the presence of additional thrombotic risk factors, consider transdermal route of administration |
| Chronic respiratory disease that may be worsened by erythrocytosis/polycythemia | Consider transdermal route of administration, and/or low dose/slow titration with monitoring. Consider referral to respirology |
| Severe/uncontrolled sleep apnea | Initiate CPAP or oral device, refer to dietitian/ encourage lifestyle changes if overweight, monitor for changes in CPAP pressure requirements. More information can be found in the full Guidelines |
Immunologic
More information on HIV in the full Guidelines.
| Risk factors | How to minimize risks |
|---|---|
| Autoimmune conditions (e.g. RA, MS, IBD) | Consider low dose/slow titration with monitoring in collaboration with any involved specialists |
| HIV | Screen patients who are at risk and treat as per current national guidelines, pay particular attention to CVD and osteoporosis risk reduction, consider use of PrEP in HIV negative patients who are at risk |
Legend for short forms:
- BP: blood pressure; NAFLD: non-alcoholic fatty liver disease; DVT: deep vein thrombosis; PE: pulmonary embolus; CPAP: continuous positive airway pressure; NRT: nicotine replacement therapy; RA: Rheumatoid arthritis; MS: multiple sclerosis; IBD: inflammatory bowel disease
Keep in mind
Denying access to hormones can do much more harm than the possible risks of treatment. With an informed-consent approach (support, information, education and consent) many providers will initiate hormones with individuals with higher risk profiles when it increases their quality of life/chance of survival greatly.Monitoring and dose titration strategies
Standard monitoring of testosterone administration should be employed at baseline, and at 3, 6, and 12 months following initiation. Some clinicians prefer to see patients monthly until an effective dose is established. Follow up visits should include a functional inquiry, targeted physical exam, blood work, and health promotion/disease prevention counselling as indicated.
Titration of doses will generally occur in the early phases of treatment. For example, with injectable testosterone, a starting dose of 30mg injected weekly could be increased by 10-20mg every 4-6 weeks. Speed of titration will depend on lab results, patient goals, response, and side effects. There may be utility in varying the timing of blood work to gather information regarding serum levels throughout the cycle (peak, mid-cycle, and trough), especially if a patient is reporting cyclic symptoms. Some patients will intentionally seek testosterone levels mid-way between the male and female range. For patients seeking maximum masculinization, the target dose will bring the testosterone level into the physiologic male range. Once the midpoint of the male reference range is attained, additional benefit is questionable. Supraphysiologic levels should be avoided due to the increased risk of adverse events and side effects, as well the potential for the aromatization of excess testosterone into estrogen. Dose reduction is warranted if supraphysiologic doses are measured at mid-cycle or trough. There may be some irregular bleeding or spotting in the first few months of treatment. However, once sustained menstrual cessation is achieved, any vaginal bleeding without explanation (e.g. missed dose(s) or lowered dose of testosterone) warrants a full workup for endometrial hyperplasia/cancer.
Keep in mind
- Clinical effects are the goal of therapy, not specific lab values.
- For lab results, reference ranges will refer to the sex assigned at birth if the sex marker associated with the patient’s health card has not been changed. Reference ranges vary between laboratories - refer to reference ranges from the specific laboratory (often available online or by request from the lab).
Reference Ranges (Lifelabs)
Hormone Monitoring Summary
See tables below for a summary of recommended monitoring for transmasculine patients at baseline, 3, 6, and 12 months after starting therapy
Note: Some providers prefer to see patients monthly until an effective dose is established.
| Baseline (See Planning Period Checklist for complete list) | Month 3 | Month 6 | Month 12c | Yearly | |||
|---|---|---|---|---|---|---|---|
| Review |
|
|
Please see the Appendix F and G of the Guidelines for a Preventive Care Checklist for transmasculine Patients and accompaniment. | ||||
| Exam
/ Investigation |
|
|
|||||
| Immunizations |
|
||||||
Summary of recommended labs
Note: In this table, lighter grey checkmarks indicate parameters that are measured under particular circumstances.
| Baseline | Month 3 | Month 6 | Month 12c | Yearly | According to guidelines for cis patients, or provider discretion | |
|---|---|---|---|---|---|---|
| CBCa | ✔ | ✔ | ✔ | ✔ | ✔ | |
| ALT | ✔ | ✔d | ✔ | |||
| HbA1c or Fasting Glucose | ✔ | ✔d | ✔ | |||
| Lipid profile | ✔ | ✔d | ✔ | |||
| Total testosterone | ✔ | ✔ | ✔ | ✔ | ✔ | |
| LHb | ✔ | ✔ | ✔ | Other | Hep B,C Pregnancy test (before first dose) |
|
| Consider HIV, syphilis, and other STI screening as indicated, frequency depending on risk | ||||||
Mental health
Suggested elements for review by Collaborative MD and Nursing Team
- Screen for mood changes including irritability/anger, depressive symptoms (including suicidality), anxiety, hypomania/mania and psychotic symptoms in those with underlying predisposition
- Inquire re: current experience of gender dypshoria/incongruence and body image
- Screen for disordered eating
- Assess patient interest in surgical treatments if not previously undergone
- Inquire re: libido/changes in libido
Education / Lifestyle counselling
Suggested elements for review by Collaborative MD and Nursing Team
- Review healthy eating and general nutrition
- Adequate Calcium Intake – ensure a minimum intake of 1200 mg of Calcium daily (total: diet + supplements)
- Adequate Vitamin D – ensure 1000 IU of vitamin D daily
- Hormone Adherence – missed doses of testosterone may impact bone health if post-oophorectomy
- Regular, moderate physical activity – encourage weight-bearing exercise for osteoporosis prevention; to avoid tendon rupture, weight loads used in strength training should be increased gradually with an emphasis on repetitions and stretching
- Safe sex practices/STI counselling – transmasculine patients may be at high risk of STIs depending on behavioural factors; safer sex counselling is recommended (for patient-centred handout materials, see PRIMED2: The back pocket guide for trans men and the men who love them
- Potential for pregnancy/need for birth control – transmasculine patients on testosterone may become pregnant even if menstrual suppression has been achieved and should be counselled in this regard; given that testosterone is a teratogen, reliable birth control should be instituted where pregnancy is a risk based on sexual activity
- Smoking – cessation, stages of readiness, motivational interviewing, etc.
- Alcohol and other substances – inquire re: problematic use of substances including non-prescribed hormones or anabolic steroids; due to presumed smaller liver size we recommend transmasculine patients follow the safe drinking guidelines as for cis women (see Canada’s Low-risk Alcohol Drinking guidelines)
Psychosocial
Suggested elements for review by Collaborative MD and Nursing Team
- An effort should be made to assess the impact of transition/trans identity on employment, housing, family, relationships, and economic wellbeing
- Social Supports – specific attention should be given to assessing the extent of a patient’s social supports, creating an opportunity to suggest additional resources if needed
- Name change/identification – assess patient need/ desire to change name and/or gender marker on identification and offer support for this process (see template letters and RHO fact sheets in the resource section)
Health maintenance
Suggested elements for review by Collaborative MD and Nursing Team
- Immunization history
- STI screening, HIV risk assessment and screening as indicated, frequent screening (i.e. every 3 months) for those at high risk; consider indications for HIV PrEP
- TB skin test as indicated
Focused Physical Exam with PAP if indicated
Suggested elements for review by Collaborative MD and Nursing Team
- Follow cervical cancer screening guidelines as for cis women if the cervix is present. For more information see next section on long-term preventive care.
- Several strategies may be employed to minimize the discomfort/trauma associated with this examination for some transmasculine patients (see RHO's Tips for Providing Paps to Trans men)
- Barring contraindications, topical 2% lidocaine jelly may be applied vaginally 5-10 minutes prior to the procedure in those who find speculum examination painful due to atrophic changes
Additional notes and references
- NB Individual parameters should be considered more frequently if concerns identified or existing risk factors are present
- a) Male reference ranges should be used for Hb/Hct (lower limit of female range can be used if menstruating)
- b) Post-gonadectomy only: elevated LH may have implications regarding bone mineral density (See full Guidelines for Osteoporosis and BMD Screening)
- c) During first year of treatment only
- d) Once at either 6- or 12-month mark
Long-term preventive care
Transmasculine patients maintained on masculinizing hormone therapy have unique preventive care needs and recommendations.
Long-term follow-up care of transmasculine patients on masculinizing hormone therapy should involve (at least) annual preventive care visits. An Adaptive Preventive Care Checklist with accompanying explanations for trans-specific recommendations can be accessed below.
-
Preventive care checklist for transmasculine patients
-
Accompaniment to preventive care checklist for transmasculine patients
Ensuring patient comfort in clinical encounters
Physical examinations that involve intimate body parts are discomforting to anyone. However, it is important to consider how trans patients may need a slightly different approach in some areas of primary care practice: tasks like disease prevention and screening, which may also require us to think differently.
It is important to reflect on approaches to clinical encounters with trans patients (e.g., consider offering to use a side- lying rather than lithotomy position when doing a Pap test with transmasculine individuals) to provide a more comfortable and gender-affirming experience.
When interacting with trans patients, asking what’s most comfortable for them is fundamental—what one patient prefers is not always transferable to the next.
Provide care based on organs present
It is best to base routine screening on the presence or absence of body parts. Refrain from calling body parts ‘male’ or ‘female’. Instead use non-gendered terms or ask the patient what they prefer to call their body parts. Organs present should receive routine preventive care.
Click on one of the tabs to find out about routine care and screening suggestions
Osteoporosis and Bone Mineral Density Screening
Indications for BMD screening
- Patients over 65 years old
- Patients 50-64 years old at higher risk for osteoporosis (e.g. smoking history, HIV+, high alcohol intake, body weight under 60 kg)
- Consider before 50 years old:
- Certain high-risk conditions such as hyperparathyroidism or malabsorption syndrome.
- Patients who have undergone oophorectomy and have been on low-dose or no exogenous testosterone for any significant length of time (>2 years).
- Patients post-oophorectomy with elevated LH levels.
- Patients who have been on a GnRH analogue for a significant length of time (2 years) without co-administration of exogenous testosterone.
- All transmasculine patients should ensure a daily intake of 1000 IU Vitamin D and 1200mg of Calcium (total of diet + supplements). Gradual weight-bearing exercises should also be encouraged (this could include walking and low-impact aerobic exercise).
Keep in mind:
- There are no studies to guide the interpretation of BMD results and fracture risk in trans people, and whether to use sex assigned at birth or affirmed gender. One option is to interpret results in comparison to both men and women.
- Frequency of BMD screening will depend on the results of the initial scan
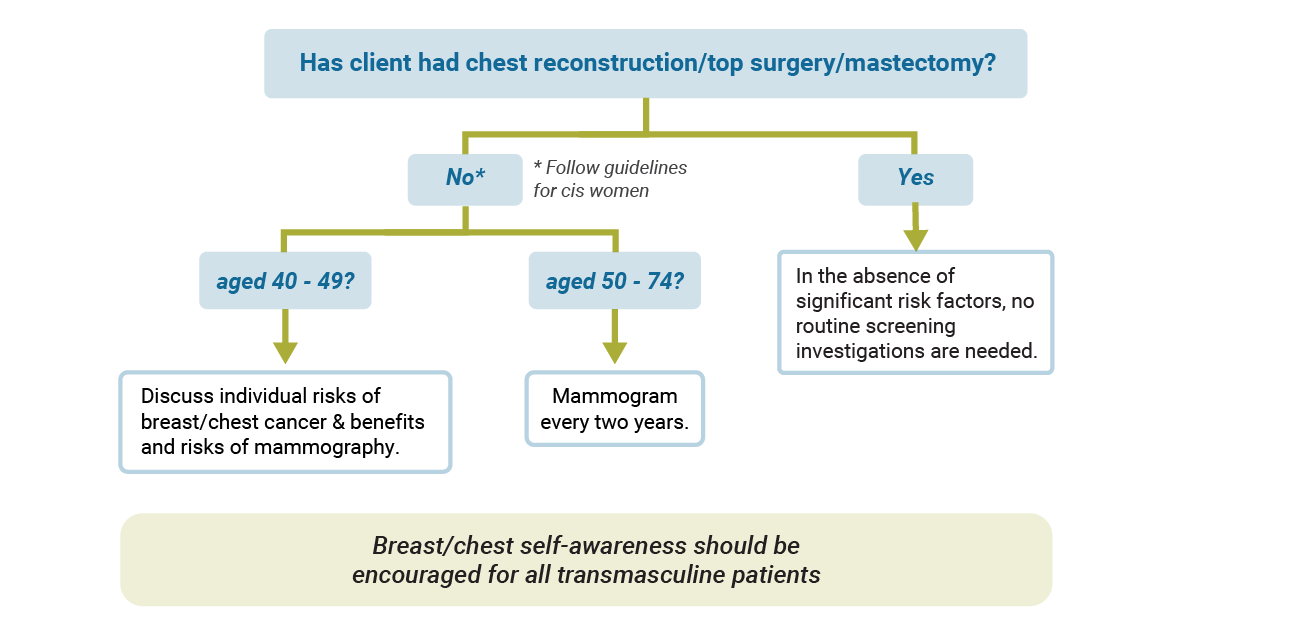
Breast/Chest Cancer Screening
Without top surgery/chest reconstruction:
Transmasculine patients who have not undergone chest reconstruction should follow guidelines for screening mammography as for cis women. As with cis women, some transmasculine patients may be at higher risk based on factors such as genetic mutations, family history and prior radiation exposure.
Following top surgery/chest reconstruction:
Transmasculine patients who have undergone chest reconstruction likely experience significant risk reduction as there is much less tissue present, however residual breast tissue does remain, particularly in the axillary regions. Mammography may not be possible following chest reconstruction. Ultrasound or MRI may be considered as alternate modalities for screening in patients with significant risk factors (or if an abnormality is detected on exam).
Keep in mind the following:
- Some transmasculine patients may prefer to use the term chest over breast(s).
- Transmasculine patients who have a male gender marker on their health card, will not be able to self-refer to the Ontario Breast Screening Program. A requisition is required in this case.
Recommendations for patients at average risk of developing breast cancer are visualized in the image below
Colon cancer screening
Screening guidelines for transmasculine patients are no different than for cis populations. Please follow your local screening guidelines (e.g. Cancer Care Ontario for Ontario guidelines).
Cervical cancer screening
Use the diagram below to find out what type of cervical cancer screening is recommended.
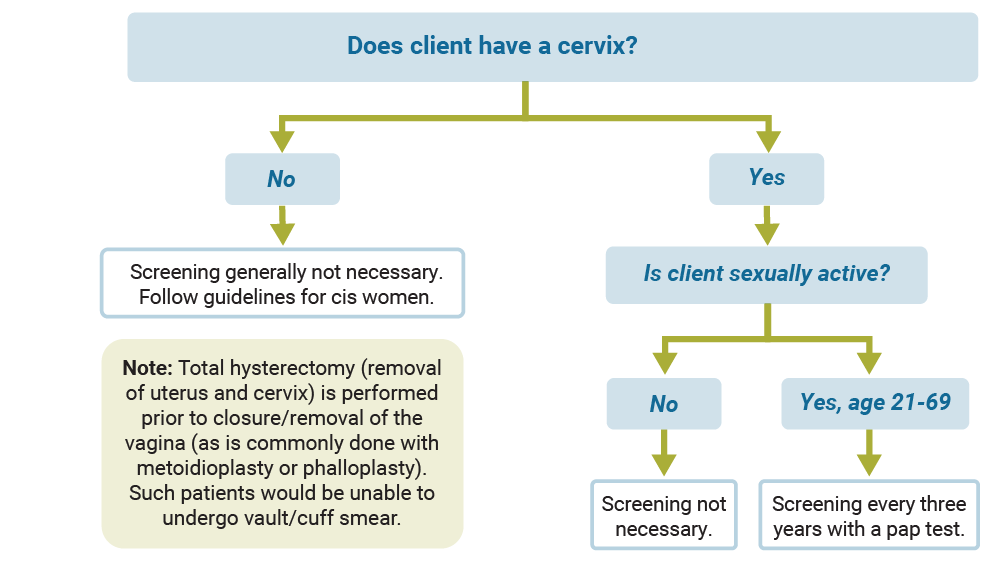
Keep in mind the following:
- Gauge your patient’s comfort with the exam. Some, but not all, transmasculine patients may feel uncomfortable with the idea of penetration and may feel their gender is undermined by the function of the speculum. It may be helpful to explain why a speculum is needed.
- Ask if the patient has had a Pap test before, or whether they have had vaginally penetrative/frontal sex. If the patient has no experience with penetration, it may be helpful to know this in advance and to suggest that they try penetration at home first using a small toy, fingers or a speculum. This may help make the screening process less confusing or disturbing. Some transmasculine patients may be willing to try this, while others will not.
- Some transmasculine patients who are taking testosterone will have fewer secretions, so using lubrication and warm water can be helpful in speculum insertion. Barring contraindications, topical 2% lidocaine jelly may be applied vaginally 5-10 min prior to the procedure in those who find speculum examination painful due to atrophic changes.
- Testosterone can cause cervical cell changes that may make the sample more difficult to read. If the patient is on testosterone, note this on the requisition.
- Inadequate samples are common in patients on testosterone.1 The use of both brush and broom may increase yield in patients with atrophic changes.2
- HPV vaccine should be offered to patients under age 45.
Source
- Peitzmeier, Sarah M., MSPH|Khullar, Karishma, BS|Reisner, Sari L., ScD, MA|Potter,Jennifer, M.D. Pap Test Use Is Lower Among Female-to-Male Patients Than Non-Transgender Women. Am J Prev Med 2014;47(6):808-812.
- Davis-Devine S, Day SJ, Anderson A, French A, Madison-Henness D, Mohar N, et al. Collection of the BD SurePath Pap Test with a broom device plus endocervical brush improves disease detection when compared to the broom device alone or the spatula plus endocervical brush combination. CytoJournal. 2008; 6:4.
- Potter J, Peitzmeier SM, Bernstein I, Reisner SL, Alizaga NM, Agénor M, et al. Cervical Cancer Screening for Patients on the Female-to-Male Spectrum: a Narrative Review and Guide for Clinicians. Journal of General Internal Medicine 2015;30(12):1857-1864.
Ovarian cancer
Analogous to concerns of an increased risk of ovarian cancer in cis women with elevated androgen levels, it has been postulated that testosterone therapy in transmasculine patients may increase risk. There have been a few cases of ovarian cancer in transgender men. Overall there is no evidence to suggest an increased risk of ovarian cancer in transmasculine patients on testosterone, and thus no cause to perform oophorectomy in transmasculine patients solely for purposes of primary prevention.