Feminizing Hormone Therapy
The goal of hormone therapy in transfeminine patients is to reduce the endogenous effects of testosterone such as a coarse body hair and facial hair; and to induce feminine secondary sex characteristics such as breast and hip development, in keeping with the patient’s individual goals. Physiologically, this requires a suppression of endogenous androgens and the addition of estrogen.
Quick reference guide for feminizing hormone therapy:
Quick reference guide for primary care providers (ENGLISH VERSION)
Aide-mémoire pour professionnels de la santé de première ligne (FRENCH VERSION)
Hormonal agents
- The anti-androgens typically used at Sherbourne Health are spironolactone and cyproterone, with the former historically chosen preferentially as it was believed to have a superior safety profile. This practice has changed somewhat over time, as adequate anti-androgen effects and testosterone suppression into the female range have been shown to be attainable at lower doses of cyproterone (i.e. 12.5 – 25 mg daily) at which adverse effects are less likely. Thus the choice of anti-androgen should be made individually for each patient based on their medical history and preference regarding risk and side effect profiles.
- If contraindications or intolerances exist for both spironolactone and cyproterone, GnRH analogs (leuprolide or “Lupron”) may be considered. Finasteride is a less effective anti-androgen and is generally not recommended, but may be considered for those who desire very mild anti-androgenic effects (dose range 1-5 mg daily).
- Following orchiectomy (+/- vaginoplasty), most transfeminine patients will not require androgen suppression. The androgen-blocker can be stopped immediately after surgery or tapered over the course of 4-6 weeks or more depending on risk factors (e.g. in patients with hypertension or renal dysfunction on spironolactone consider monitored taper).
For help deciding which anti-androgen may be best for an individual patient, please refer to the following table comparing effects, side effects, and contraindications of spironolactone and cyproterone.
- Estrogen acts directly on estrogen receptors to initiate feminization. Several forms and routes of estrogen have been used for feminization. At Sherbourne Health, the most common form used is oral 17-β estradiol (Estrace), which is now covered by the ODB program without an EAP request. While conjugated estrogens (e.g. Premarin) have historically been used due to their accessibility/affordability, they are no longer recommended.1
- There is a lack of consensus on the preferred timing of the initiation of estrogens in relation to an anti-androgen. Common approaches have included both the initiation of an anti-androgen (usually 1-3 months) prior to the addition of estrogen, or alternatively, the simultaneous introduction and subsequent titration of both components. The starting dose of estrogen can be maintained for 1-2 months, after which a dose increase can be considered barring any concerning effects. In patients over 50 years old who have been on estrogen for several years, doses may be reduced to those administered to post-menopausal cis women (e.g. starting/low dose topical formulations, i.e. 0.025 – 0.05 mg patch).
- With the exception of cyproterone, the use of progestins in transfeminine patients continues to be controversial2. There have been anecdotal reports of improved breast and/or areolar development, mood, sleep, and libido with the use of progestins3,4; however a clear impact has yet to be demonstrated. Common side effects include weight gain, edema and depression. Given the lack of clear benefit, and potential risks, progestins are not routinely recommended as part of a feminizing hormone regimen. However, should patients request progestins, a trial may be considered following a frank discussion of expectations and risks. Further information can be found in the full Guidelines, and in the Checklist for Patient Review - Initiation of Progestin Therapy.
- 2. Hembree WC, Cohen-Kettenis P, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 2017
- 3. Wierckx K, Gooren L, T'Sjoen G. Clinical Review: Breast Development in transfeminine patients Receiving Cross‐Sex Hormones. The Journal of Sexual Medicine 2014;11(5):1240-1247.
- 4. Orentreich N, Durr NP. MAMMOGENESIS IN TRANSSEXUALS. J Invest Dermatol 1974;63(1):142-146.
Sources
| Formulations | Starting Dose | Usual Dose | Maximum Dose | Cost*(4 weeks) | |
|---|---|---|---|---|---|
| ANTIANDROGENS | Spironolactone (oral) | 50mg daily - BID | 100 mg BID | 150 mg bida | $15 - $41 |
| Cyproterone (oral) | 12.5 mg (1/4 50 mg tab) q2d - daily | 12.5 mg (1/4 50 mg tab) – 25 mg (1/2 50 mg tab) daily | 50 mg dailya | $16 - $56 | |
| ESTROGENS | Estradiol (oral)* | 1-2mg daily | 4 mg daily or 2 mg bid | 6 mg daily or 3 mg BID | $18 - $54 Covered by ODB |
| Estradiol (transdermal, patch)*b | 50 mcg daily/apply patches 2x/week | Variablec | 200 mcg daily/apply patch 2x/week | $39 - $76d | |
| Estradiol (transdermal, gel)**e | 2.5 g daily (2 pumps, contains 150 mcg estradiol) | Variablec | 6.25 g daily (5 pumps, contains 275 mcg estradiol), may be limited by surface area requirements for gel application | $58 - $154 | |
| Estradiol Valerate**Injectable (IM)f | 3-4 mg q weekly or 6-8 mg q 2 weeks | Variablec | 10mg q weekly | $36 - $46 | |
References and Notes
|
|||||
Keep in mind
If a patient is taking IM estrogen and wishes to self-inject, it is important to instruct them on the technique for safe injection and sharps disposal. A written step-by-step guide on self-injection for patients is available from Fenway Health.Expected effects
What to expect from a regimen consisting of an anti-androgen and estrogen
The degree and rate of physical effects are largely dependent on patient-specific factors such as age, genetics, body habitus and lifestyle, and to some extent the dose and route used (selected in accordance with a patient’s specific goals and risk profile). Physical changes related to androgen blockade and estrogen may take months to appear and are generally considered to be complete after 2-3 years on hormone therapy. Breast growth is an aspect of feminization to which many transfeminine patients assign great importance. The degree of breast development is dependent on many factors, but most transfeminine patients experience modest breast development (average cup size < A, or developmental Tanner stage 2 to 3).12) In early studies, neither type nor dosage of estrogen was shown to affect final breast size, and no relationship between serum estradiol levels and breast development was found.12, 13
Feminizing therapy does not affect the pitch of the voice in transfeminine patients. Some patients may obtain benefit from voice therapy with a qualified and supportive speech and language therapist who can work with the patient to modify their vocal characteristics. There are also a variety of surgical techniques (not covered by OHIP) that have been utilized to feminize the voice through alteration of the vocal cords.
Sources
- 12 Wierckx K, Gooren L, T'Sjoen G. Clinical Review: Breast Development in transfeminine patients Receiving Cross‐Sex Hormones. The Journal of Sexual Medicine 2014;11(5):1240-1247
- 13de Blok C, Klaver M, Wiepjes CM, Nota NM, Heijboer AC, Fisher AD, et al. Breast Development in Transwomen After 1 Year of Cross-Sex Hormone Therapy: Results of a Prospective Multicenter Study. JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM 2018;103(2):532-538.
Keep in mind
Hormone therapy results in both reversible and irreversible feminizationEffects and expected time course
Sources
- Adapted from Hembree et al., The Endocrine Treatment of Gender-Dysphoric/Gender Incongruent Persons: An Endocrine Society Guideline
- a) Estimates for onset and expected maximum effect represent published and unpublished clinical observations. Sources:
- Toorians AWFT, Thomassen MCLGD, Zweegman S, Magdeleyns EJP, Tans G, Gooren LJG, et al. Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people. J Clin Endocrinol Metab. 2003 Dec;88(12):5723–9.
- Asscheman H, Gooren LJ, Assies J, Smits JP, de Slegte R. Prolactin levels and pituitary enlargement in hormone-treated male-to-female transsexuals. Clin Endocrinol (Oxf). 1988 Jun;28(6):583–8.
- Gooren LJ, Harmsen-Louman W, van Kessel H. Followup of prolactin levels in long-term oestrogen-treated male-to-female transsexuals with regard to prolactinoma induction. Clin Endocrinol (Oxf). 1985 Feb;22(2):201–7.
- b) Significantly dependent on amount of exercise
- c) Complete removal of facial hair requires electrolysis, laser treatment, or both
- Visual reference: Tetzlaff K. Patient’s guide to transgender, trans, & gender diverse health. 2015.
Risk mitigation
Contraindications
- Unstable ischemic cardiovascular disease
- Estrogen-dependent cancer
- End stage chronic liver disease
- Psychiatric conditions which limit the ability to provide informed consent
- Hypersensitivity to one of the components of the formulation
Precautions and risk mitigation
Pre-existing medical conditions and risk factors may impart increased risks with estrogen administration and should be considered in order to enable individualized discussions with patients regarding their unique risks and benefits of treatment. Available measures to reduce associated risks should be considered and discussed with patients and, if possible, undertaken prior to or concurrently with the initiation of hormone therapy.
Precautions in red impart moderate to high risk of an adverse outcome without risk mitigation
Select area of concern below
Neurologic
More information on seizure disorders and anticonvulsant therapy is in the full Guidelines
| Risk factors | How to minimize risks |
|---|---|
| Cerebrovascular disease | Consider referral to neurology, ensure optimal medical management (including prophylactic anti-platelet agent(s) if indicated per current national guidelines) and risk factor optimization, use transdermal route of administration +/- lower dose |
| Severe refractory or focal migraine | Consider referral to neurology, consider daily migraine prophylaxis, ensure all other cerebrovascular risk factors are optimized, consider transdermal route of administration, consider spironolactone as preferred anti-androgen |
| Seizure disorders | Consider referral to neurology, consult with a pharmacist re: possible estrogen interaction with anticonvulsant medication |
| History of benign intracranial hypertension | Consider referral to neurology/neurosurgery |
Endocrine
More information on metabolic risk factors and hyperprolactinemia/prolactinoma in the full Guidelines
| Risk factors | How to minimize risks |
|---|---|
| Hyperprolactinemia | Determine etiology and manage as indicated, if prolactin >80mcg/L or symptomatic – rule out prolactinoma, refer to endocrinology as needed, consider spironolactone as preferred anti-androgen |
| Marked hypertriglyceridemia | Identify and address barriers to optimal lipid control, refer to dietician, minimize alcohol consumption, consider anti-lipemic pharmacologic therapy, consider endocrinology referral, use transdermal route of administration |
| Uncontrolled diabetes | Identify and address barriers to optimal glycemic control, refer to dietitian, encourage lifestyle modification, initiate antiglycemic agent(s) per national guidelines, consider cardiac stress test, consider transdermal route of administration |
| Metabolic syndrome | Dietary and medical management of component disorders, consider cardiac stress test, consider transdermal route of administration |
Cardiovascular
More information on cardiovascular disease and related metabolic risk factors in the full Guidelines
| Risk factors | How to minimize risks |
|---|---|
| Stable ischemic cardiovascular disease | Consider referral to cardiology, ensure optimal medical (including prophylactic anti-platelet agent(s) if indicated per national guidelines) and/or surgical management as indicated, risk factor optimization, use transdermal route of administration +/- lower dose, consider spironolactone as preferred anti-androgen |
| Other cardiac diseases | Consider referral to cardiology |
| Uncontrolled high blood pressure | Identify and address barriers to optimal BP control, use spironolactone as anti-androgen, add additional antihypertensives as needed (avoid ACEs/ARBs with spironolactone), consider cardiac stress test, consider transdermal route of administration |
Hepatic
More information on liver/gallbladder effects in the full Guidelines
| Risk factor | How to minimize risks |
|---|---|
| Hepatic dysfunction | Dependent on etiology, eg. minimize alcohol consumption, weight loss in NAFLD, consider referral to hepatology/GI, use transdermal, sublingual, or injectable route of administration, consider spironolactone as preferred anti-androgen |
| Hepatitis C | Screen patients who are at risk and treat as per current national guidelines !
|
Oncologic
More information on breast cancer in the full Guidelines
| Risk factors | How to minimize risks |
|---|---|
| Strong family history of breast cancer | Refer to genetics/familial breast cancer program for further risk stratification and genetic testing as indicated |
| Prior history of estrogen-sensitive cancer | Refer to oncology |
Hematologic
More information on venous thromboembolism in the Guidelines
| Risk factors | How to minimize risks |
|---|---|
| Personal or family history of porphyria (rare) | Consider referral to porphyria clinic or internist with experience in porphyria |
| Hypercoagulable state or personal history of deep vein thrombosis (DVT) or pulmonary embolism (PE) | Identify and minimize existent risk factors, prophylactic anti-platelet agent(s) if indicated per current national guidelines, consider referral to hematology/thrombosis clinic, use transdermal route of administration +/- lower dose, consider spironolactone as preferred anti-androgen |
| Strong family history of abnormal clotting | Rule out genetic clotting disorder, consider transdermal route of administration, consider spironolactone as preferred anti-androgen |
Respiratory
| Risk/Precaution | How to minimize risks |
|---|---|
| Smoker | Encourage and support smoking cessation, consider referral to smoking cessation program/offer NRT and/or bupropion/varenicline, or negotiate a decrease in smoking, consider cardiac stress test, use transdermal route of administration +/- lower dose, consider spironolactone as preferred anti-androgen, consider low-dose ASA prophylaxis |
Immunologic
More information on HIV in the Guidelines
| Risk/Precaution | How to minimize risks |
|---|---|
| Autoimmune conditions (e.g. RA, MS, IBD) | Start low dose, titrate slowly in collaboration with any involved specialists |
| HIV | Screen patients who are at risk and treat as per current national guidelines, use caution with concomitant use of spironolactone and septra (for prophylaxis of opportunistic infections) due to risk of severe hyperkalemia, pay particular attention to CVD and osteoporosis risk reduction, consider use of PrEP in HIV negative patients who are at risk |
Legend for short forms:
- ACE/ARB: angiotensin converting enzyme inhibitors/angiotensin-receptor blockers; ASA: acetylsalicylic acid; BP: blood pressure; NAFLD: non-alcoholic fatty liver disease; DVT: deep vein thrombosis; PE: pulmonary embolus; NRT: nicotine replacement therapy; RA: Rheumatoid arthritis; MS: multiple sclerosis; IBD: inflammatory bowel disease; HIV: Human Immunodeficiency Virus
Keep in mind
In many cases, denying access to hormones can do much more harm than the possible risk of treatment. With an informed consent approach (support, information, education and consent) many providers will initiate hormones with individuals with higher risk profiles when it increases their quality of life/chance survival greatly.Monitoring and dose titration strategies
Standard monitoring of a feminizing regimen should be employed at baseline, and at 3, 6, and 12 months following initiation (creatinine and electrolytes should be checked 4-6 weeks after initiation or dose increase of spironolactone). Some providers prefer to see patients monthly until an effective dose is established. Follow up visits should include a functional inquiry, targeted physical exam, bloodwork, and health promotion/disease prevention counselling as indicated.
Dose titration of anti-androgen and estrogen may be performed over the course of 3-6 months or more and will depend on patient goals, physical response, measured serum hormone levels, and other lab results.
A common titration might look like:
STARTING DOSE
TITRATING UP
- At 1 month: Check creatinine and electrolytes at one month and, barring any concerns, increase estradiol to 2 mg and spironolactone to 50 mg twice daily;
- At 3 months: Following three-month bloodwork and check-in, increase estradiol to 3 mg daily and spironolactone to 75 mg twice daily; and
- Continue titration as needed until maintenance dose is achieved.
Hormone levels for those seeking a more androgynous appearance may intentionally be mid-range between male and female norms. For many transfeminine patients, the goal will be to achieve the suppression of testosterone into the female range (see Appendix J in the Guidelines). Be mindful that patients may have clinically relevant results without total suppression of testosterone because of of peripheral androgen blockade, which is not measured.
In the vast majority of cases, the measurement of total testosterone is adequate to assess the degree of androgen suppression. Measurements and calculated estimates of free testosterone are imprecise and generally don’t add value.
Serum estradiol levels should also be monitored. Anecdotally, we have found that most patients reach considerable feminization at estradiol levels between 200-500 pmol/L.
Keep in mind
- Clinical effects are the goal of therapy, not specific lab values.
- For lab results, reference ranges will refer to the sex assigned at birth if the sex marker associated with the patient’s health card has not been changed. Reference ranges vary between laboratories - refer to reference ranges from the specific laboratory (often available online or by request from the lab).
APPENDIX J: Reference Ranges (Lifelabs)
Click here to view monitoring parameters specifically for spironolactone or cyproterone without estrogen
Hormone Monitoring Summary
See tables below for a summary of recommended monitoring for transfeminine Patients at baseline, 3, 6, and 12 months after starting therapy
Note: Some providers prefer to see patients monthly until an effective dose is established.
| Baseline (See Planning Period Checklist for complete list) | Month 3 | Month 6 | Month 12e | Yearly | |
|---|---|---|---|---|---|
| Review |
|
|
Please see Appendix D of the Guidelines for a Preventive Care Checklist for Transfeminine Patients and Accompanying Explanations | ||
| Exam
inations/ investigations |
|
|
|||
| Immunizations |
|
Notes | *for patients who may have interest in OHIP-covered breast augmentation surgery, breast inspection at baseline and 12 months with particular attention to Tanner stage. Chest circumference at fullest part of the breast and areolar diameter may be helpful in determining the presence or absence of breast growth | ||
Summary for recommended labs
Note: In this table, lighter grey checkmarks indicate parameters that are measured under particular circumstances.
| Baseline | 3 months | 6 months | 12 monthse |
Yearly | According to guidelines for cis patients, or provider discretion | |
|---|---|---|---|---|---|---|
| CBCa | ✔ | ✔ (if on cyproterone) |
✔ (if on cyproterone) |
✔ (if on cyproterone) |
✔ (if on cyproterone) |
|
| ALTb | ✔ | ✔ (if on cyproterone) |
✔ (if on cyproterone) |
✔ | ✔ | ✔ |
| Creatinine/Lytesc | ✔ | ✔ (if on spironolactone) |
✔ (if on spironolactone) |
✔ | ✔ (if on spironolactone) |
|
| HbA1c or Fasting Glucose | ✔ | ✔ | ✔ | |||
| Lipid profile | ✔ | ✔ | ✔ | |||
| Total testosterone | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Estradiol | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Prolactind | ✔ | ✔ (if on cyproterone) |
✔ (if on cyproterone) |
✔ | ||
| Other | Hep B, C |
Consider HIV, syphilis, and other STI screening as indicated |
||||
Additional notes and references
- a) Baseline for all and regularly with cyproterone, otherwise repeat once at 6-12 months then as needed. For Hb/Hct use female reference for lower limit of normal and male reference for upper limit of normal.
- b) Baseline for all and regularly with cyproterone, otherwise repeat once at 6-12 months then as needed
- c) Cr/lytes should be monitored at each visit with spironolactone (including 4-6 weeks after starting and after any dose changes), but is only required at baseline and then once between 6-12 months with cyproterone unless risk factors or concerns re: renal disease are present; use male reference range for upper limit of normal for Cr
- d) Prolactin should be monitored at least yearly with the use of cyproterone, and more frequently if elevation noted
- e) During first year of treatment only
Long-term preventive care
Transfeminine patients maintained on feminizing hormone therapy have unique preventive care needs and recommendations.
Long-term care of transfeminine patients on feminizing hormone therapy should involve (at least) annual preventive care visits. A Preventive Care Checklist with accompanying explanations for trans-specific recommendations can be accessed below.
-
Preventive care checklist for transfeminine patients
-
Accompaniment to the preventive care checklist for transfeminine patients
Ensuring patient comfort in clinical encounters
Physical examinations that involve intimate body parts are discomforting to anyone. However, it is important to consider how trans patients may need a slightly different approach in some areas of primary care practice: tasks like disease prevention and screening, which may also require us to think differently.
It is important to reflect on approaches to clinical encounters with trans patients to provide a more comfortable and gender-affirming experience.
When interacting with trans patients, asking what’s most comfortable for them is fundamental—what one patient prefers is not always transferable to the next.
Provide care based on organs present
It is best to base routine screening on the presence or absence of body parts. Refrain from calling body parts ‘male’ or ‘female’. Instead use non-gendered terms or ask the patient what they prefer to call their body parts. Organs present should receive routine preventive care.
Click on one of the tabs to learn about routine care and screening suggestions.
Osteoporosis and bone mineral density screening
Indications for BMD screening
- All patients over 65 years old.
- Patients 50-64 years old at higher risk for osteoporosis (e.g. smoking history, HIV+, high alcohol intake, body weight under 60 kg).
- Consider before 50 years in:
- Certain high-risk conditions such as hyperparathyroidism or malabsorption syndrome.
- Patients who have undergone orchiectomy and have been on low-dose or no hormones for any significant length of time (>2 years).
- Patients who have been on anti-androgens or a GnRH analogue for a significant length of time (>2 years) without co-administration of exogenous estrogen.
- All transfeminine patients should ensure a daily intake of 1000 IU Vitamin D and 1200mg of Calcium (total of diet + supplements).
- Weight-bearing exercise should also be encouraged (this could include walking and low-impact aerobic exercise).
Keep in mind:
- There are no studies to guide the interpretation of BMD results and fracture risk in trans people, and whether to use sex assigned at birth or affirmed gender. One option is be to interpret results in comparison to both cis men and cis women.
- Frequency of BMD screening will depend on the results of the initial scan.
Breast/Chest health
- Longer duration of feminizing hormone exposure (i.e. number of years taking estrogen), family history of breast cancer, obesity (BMI >35), and the use of progestins likely increase the level of risk of breast cancer.1
- Transfeminine patients should receive counselling around breast self-awareness as is recommended for cis women.
- Annual clinical breast examination as part of routine breast cancer screening is of questionable utility, but may be useful in transfeminine patients to assess the degree of breast development or to assess for implant complications if the patient has undergone breast augmentation.
Source
- 1 Feldman J, Safer J. Hormone Therapy in Adults: Suggested Revisions to the Sixth Version of the Standards of Care. International Journal of Transgenderism 2009;11(3):146.
Keep in mind:
In Ontario, transfeminine patients who have changed their OHIP sex marker to “female” can be screened as part of the organized Ontario Breast Screening Program.
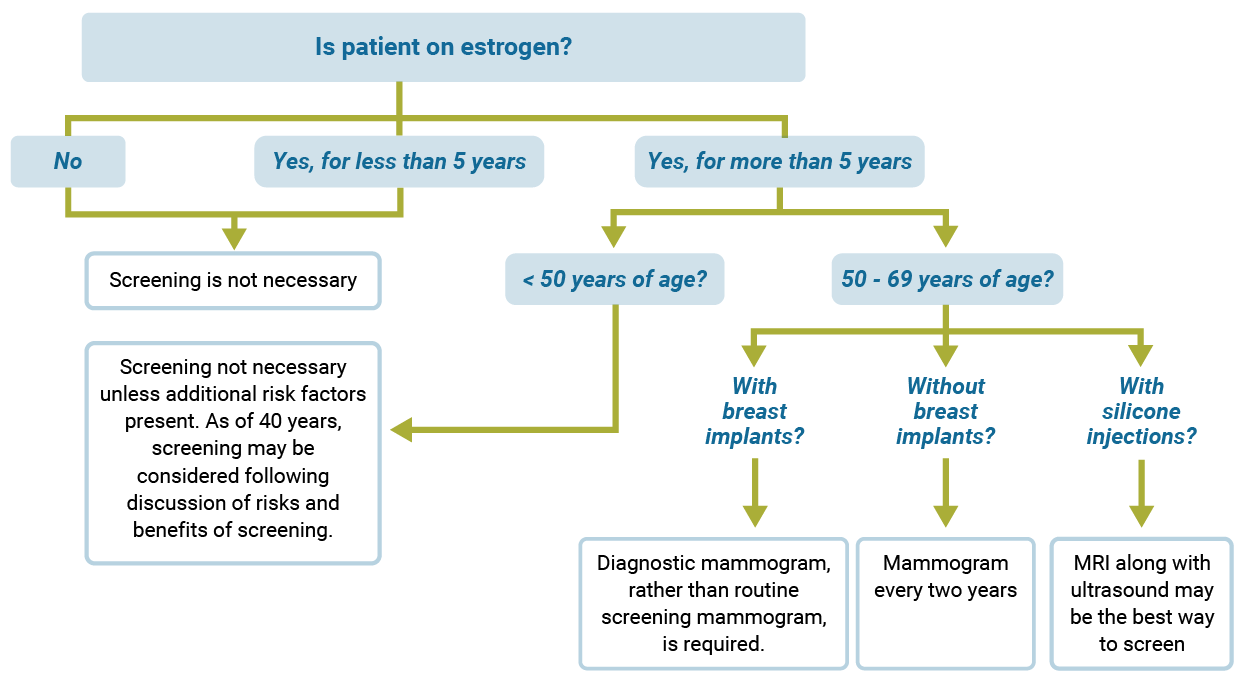
Breast Cancer Screening
Use the diagram below to find out whether your patient needs breast cancer screening. Note: This applies only to those at average risk of breast cancer (not those that have an increased risk based on genetic factors, whom should be screened earlier and more frequently).
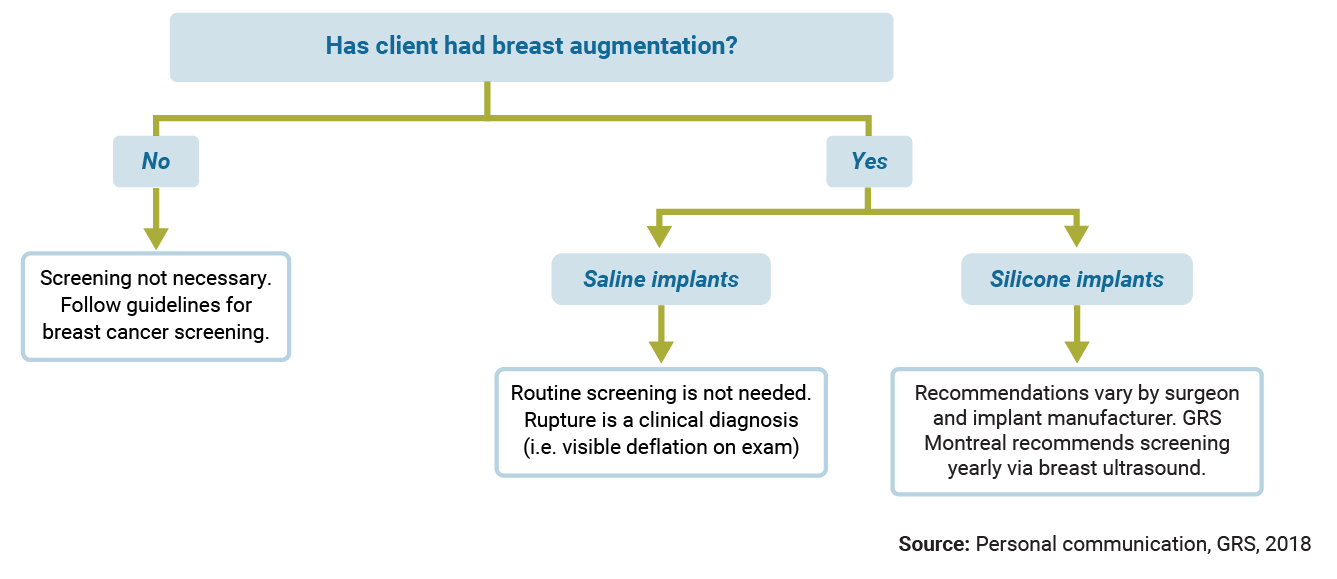
Implant Rupture Screening
Use the diagram below to find out whether your client needs screening for implant rupture.
Colon cancer screening
Screening guidelines for transfeminine patients are no different than for cis populations. Please follow your local screening guidelines (e.g. Cancer Care Ontario for Ontario guidelines).
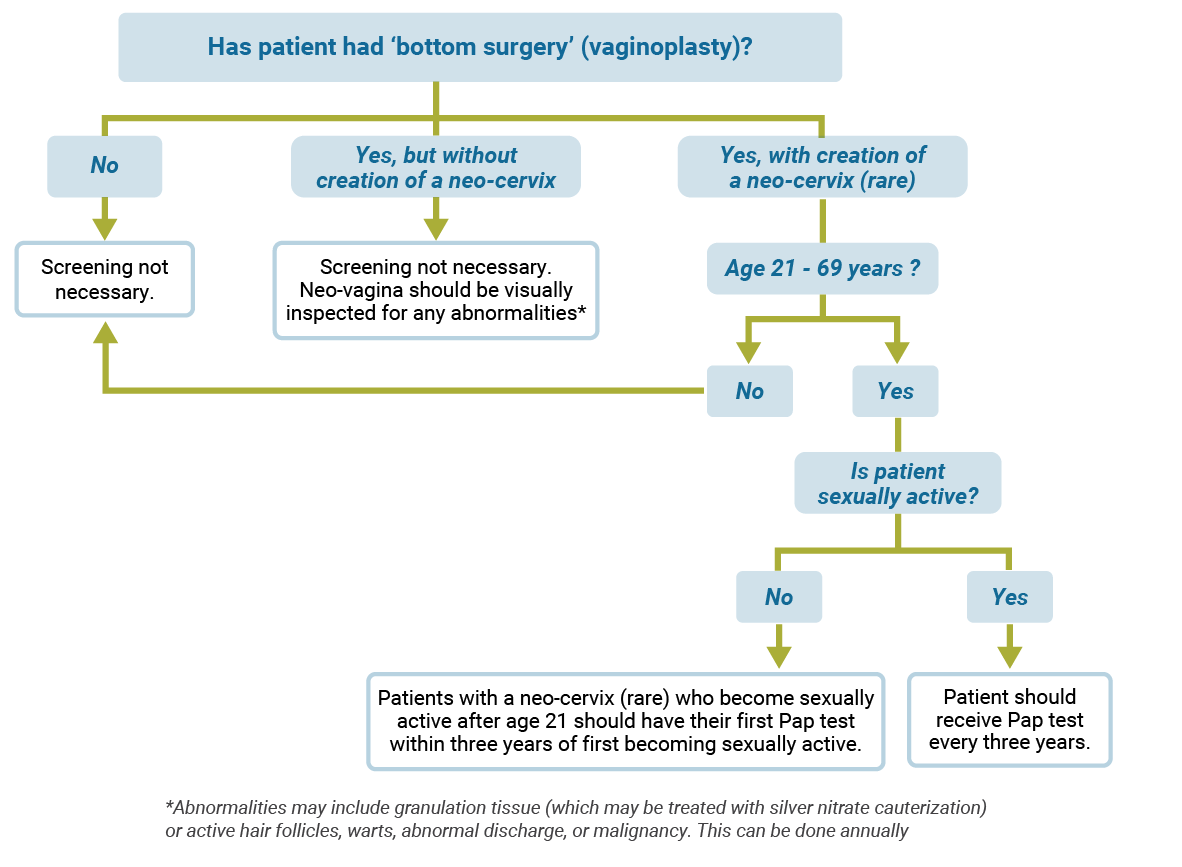
Cervical cancer screening
Use the diagram below to find out what type of cervical cancer screening is recommended.
Prostate exam
The risk of prostate cancer is not increased by estrogen use; in fact it is reasonable to assume that the risk is significantly decreased by the associated androgen deprivation. Although rare, there have been cases of prostate cancer reported in transfeminine patients, generally occurring in those who started hormone therapy after the age of 50.1, 2 It is important to note that estrogen will lower PSA values even in the presence of prostate cancer, thus impacting its utility in this population. A reduction in the upper limit of normal for PSA to 1 ng/L can be considered in transfeminine patients with low testosterone.3 Routine PSA screening is not recommended in transfeminine patients in the absence of significant risk factors. There is little evidence to support a role for annual DRE in prostate cancer screening; however, it may be considered according to a provider’s routine practice with cis men. In patients who have undergone vaginoplasty, the prostate remains in situ and may be palpated anteriorly via digital vaginal exam in a gender affirming lithotomy position.
Sources
- Gooren LJ, Giltay EJ, Bunck MC. Long term treatment of transsexuals with cross-sex hormones: extensive personal experience. J Clin Endocrinol Metab. 2008; 93(1):19-25.
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008; 159(3):197-202.
- Trum HW, Hoebeke P, Gooren LJ. Sex reassignment of transsexual people from a gynecologist’s and urologist’s persective. Acta Obstet Gynecol Scand 2015; 94(6):563-567